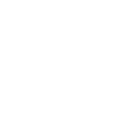Frequently Asked Questions
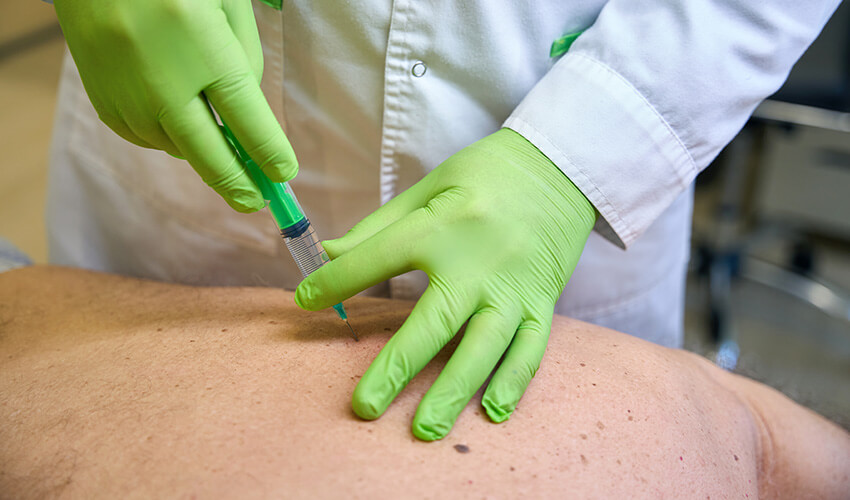
Sarapin™ is the commonly used name for the sterile compounded injectable manufactured by US Specialty Formulations. It is intended exclusively for use by licensed healthcare professionals.
Practitioner reports and historical references have included trigger point injections, joint injections, nerve block applications and other musculoskeletal procedures performed within clinical settings.
Disclaimer: Compounded Sarracenia purpurea 0.17 g/mL for injection has not been reviewed by the FDA for safety or efficacy.
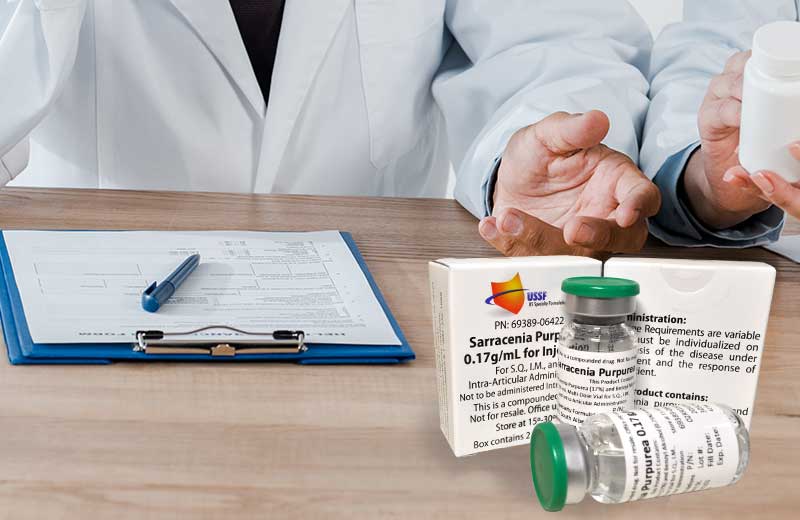
Please submit your order details for SARAPIN™ via this form: Sarapin™ Order Form. For questions about pricing, eligibility, or product details, please contact us at orderdesk@ussfgmp.com.
Compounded Sarracenia purpurea 0.17 g/mL for injection is manufactured in an FDA-registered facility with strict GMP protocols under the 503B section of the US Federal Drug and Cosmetic Act. Compounded Sarracenia purpurea 0.17 g/mL for injection has not been reviewed by the U.S. Food & Drug Administration for safety or efficacy and should only be used under the supervision of licensed healthcare professionals.
Sarapin™ is available exclusively to licensed healthcare professionals authorized to administer injections. This includes MDs, DOs, NPs, PAs, DMDs/DDSs, and chiropractors with injection privileges. Please submit your order details for SARAPIN™ via this form: Sarapin™ Order Form.
This information is provided for educational purposes only and does not constitute medical advice or imply clinical efficacy. If you are a licensed healthcare professional interested in using this product, click below to begin the verified ordering process.
Disclaimer: Compounded Sarracenia purpurea 0.17 g/mL for injection has not been reviewed by the U.S. Food & Drug Administration for safety or efficacy. It is intended for use in an office setting by licensed healthcare professionals. Availability and shipping restrictions may apply.

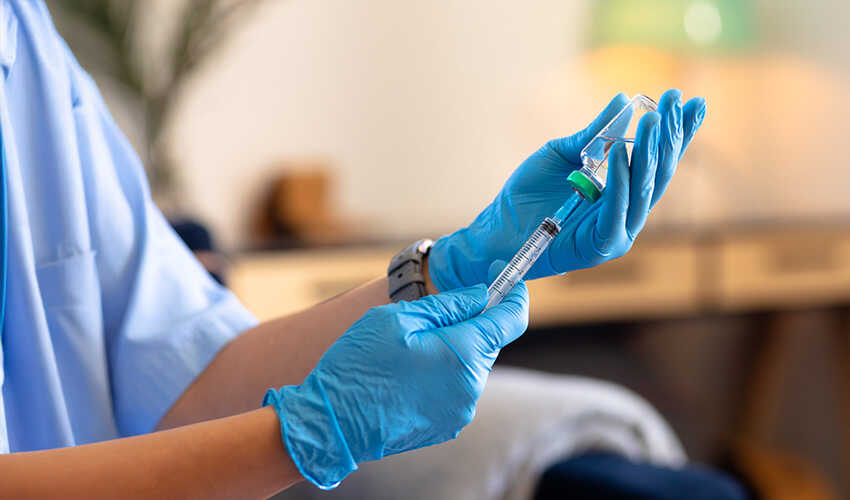




 Trigger point injections for myofascial pain
Trigger point injections for myofascial pain